
In text Questions page number 46 Chapter 3: Metal and Non-metal Science Class 10 solutions are avalable at ourwebsite to help the students. In text Questions page number 46 is solved by our expert teachers. You can get ncert solutions and notes for class 10 chapter 3 absolutely free. NCERT Solutions for class 10 Science Chapter 3: Metal and Non-metal will definitely help in improving your marks in CBSE Board examinations.
In text Questions page number 46
Question 1. Why is sodium kept immersed in kerosene oil?
Answer: Sodium is high reactive element. If it is kept in open it can explosively react with oxygen to catch fire. Hence to prevent accidental damage sodium is immersed in kerosene oil.
Question 2. Write equations for the reactions of
(i) iron with steam
(ii) calcium and potassium with water
Answer:
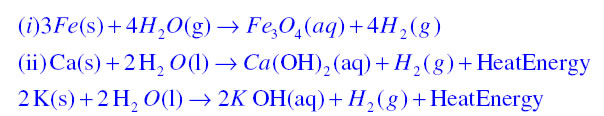
Question 3. Samples of four metals A, B, C and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows.
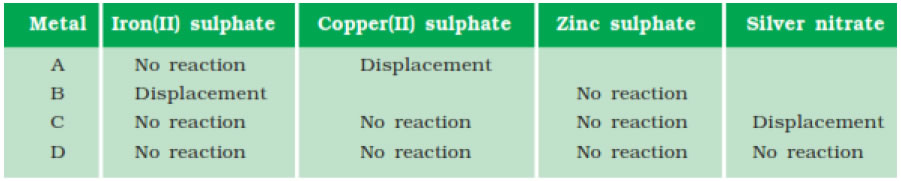
(i)Which is the most reactive metal?
Answer: B is most reactive metal.
(ii) What would you observe if B is added to a solution of Copper (II) sulphate?
Answer: B will displace copper from copper sulphate.
(iii) Arrange the metals A, B, C and D in the order of decreasing reactivity.
Answer: Arrangement of metals in the order of decreasing reactivity B>A>C>D
Question 4. Which gas is produced when dilute hydrochloric acid is added to a reactive metal? Write the chemical reaction when iron reacts with dilute sulphuric acid.
Answer: Hydrogen gas is evolved when hydrochloric acid is added to a reactive metal. The chemical reaction when iron reacts with dilute sulphuric acid is
![]()
Question 5. What would you observe when zinc is added to a solution of iron(II) sulphate? Write the chemical reaction that takes place.
Answer: When zinc is added to iron (II) sulphate then it will displace the iron from iron sulphate solution as shown in the following chemical reaction
![]()
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.